question archive Calculate the initial rate for the formation of C \rm C at 25 ? C \rm ^\circ C, if [A]=0
Calculate the initial rate for the formation of C \rm C at 25 ? C \rm ^\circ C, if [A]=0
Subject:ChemistryPrice:3.85 Bought3
Calculate the initial rate for the formation of C \rm C at 25 ? C \rm ^\circ C, if [A]=0.50M {\rm [A]}=0.50\; M and [B]=0.075M {\rm [B]}=0.075\; M. Express your answer to two significant figures and include the appropriate units Consider the reaction A+2B?C \rm A+2B \rightleftharpoons C whose rate at 25 ? C \rm ^\circ C was measured using three different sets of initial concentrations as listed in the following table: A =2, B =1 k[A]^2

Purchase A New Answer
Custom new solution created by our subject matter experts
GET A QUOTE
Answer Preview
Answer
The initial rate for the formation of reactant, C at 25 °C is 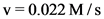 .
.
please see the attached file for complete solution.








