question archive Add electron dots and charges as necessary to show the reaction of calcium and oxygen to form an ionic compound Rank these elements according to electronegativity
Add electron dots and charges as necessary to show the reaction of calcium and oxygen to form an ionic compound Rank these elements according to electronegativity
Subject:ChemistryPrice:2.85 Bought3
Add electron dots and charges as necessary to show the reaction of calcium and oxygen to form an ionic compound Rank these elements according to electronegativity.

Purchase A New Answer
Custom new solution created by our subject matter experts
GET A QUOTE
Answer Preview
1. Neutral Ca atom has 2 valence electrons and gives away those 2 electrons to form Ca2+ ion. The neutral O atom has 6 valence electrons and it takes 2 electrons from Ca to form O2- ion. Thus calcium oxide, CaO is formed.
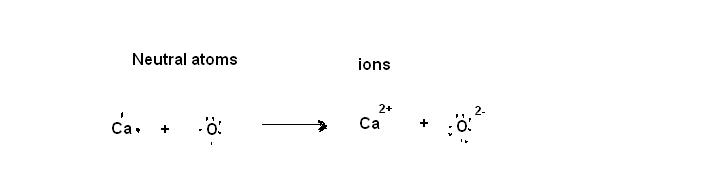
2. Electronegativity decreases down the group(from top to bottom) and increases along the period from left to right.
out of th given elements, Fluorine is the most electronegative and Cs is the least electronegative . The order of decreasing electronegativity is:
F > C > Al > Na > Cs








