question archive A) Which of the following pairs cannot be mixed together to form a buffer solution? Why? A
A) Which of the following pairs cannot be mixed together to form a buffer solution? Why? A
Subject:ChemistryPrice:2.85 Bought3
A) Which of the following pairs cannot be mixed together to form a buffer solution? Why?
A. NH3, NH4Cl
B. KOH, HF
C. NaC2H3O2, HCl
D. H3PO4, KH2PO4
E. RbOH, HCl
I already know the answer is E. I want help understanding when we can rule out choices B and C.
B) For a solution equimolar in HCN and NaCN, which statement is false?
Without giving all the choices, the false choice is "The [H+] is larger than it would be if only the HCN was in solution."
Why? How would I know this?
C) A buffer containing 0.200 M of acid, HA, and 0.150 M of its conjugate, A-, has a pH of 3.35. What is the pH after 0.015 mol of NaOH is added to 0.500 L of the solution.
How do I solve this?

Purchase A New Answer
Custom new solution created by our subject matter experts
GET A QUOTE
Answer Preview
Part 1)
A buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa
A. NH3, NH4Cl
Here, NH4Cl will dissociate in aqueous solution to form NH4+ and Cl-. Therefore, the solution contains the weak base NH3 and its conjugated acid NH4+. Thus, this combination can act as a buffer
B. KOH, HF
Here, KOH is a strong base and HF is a weak acid. KOH will consume a part of the H+ ions from HF. The resulting solution will have contained HF and F- which can act as a buffer
C. NaC2H3O2, HCl
Sodium acetate is a salt of acetic acid. In the presence of HCl, it forms acetic acid. Thus, the resulting solution will contain
sodium acetate and acetic acid which can act as a buffer
D. H3PO4, KH2PO4
Phosphoric acid is a weak acid and KH2PO4 is its potassium salt. This combination can act as a buffer
E. RbOH, HCl
RbOH is a strong base and HCl is a strong acid. This combination will not function as a buffer
Part 2)
HCN(aq) 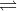 H+(aq) + CN-(aq)
H+(aq) + CN-(aq)
Thus, if NaCN is also present in the system it’ll decrease the concentration of H+ by common ion effect
Therefore,
[H+] is larger than it would be if only the HCN were in solution
Part 3)
Moles of acid = 0.2 M x 0.5 L = 100 mmoles
Moles of salt = 0.15 M x 0.5 L = 0.075 mmoles
Applying the Henderson-Hesselbalach equation
pH =pKa + log[salt]/[acid]
pH = pKa + log (75/100)
pKa = 3.35 - log (75/100) = 3.35 + 0.125 = 3.475
pKa = 3.35 + 0.125 = 3.475
pKa = 3.475
After 0.015 mol of NaOH is added
0.015 moles = 15 mmoles
The 15 mmoles of the base will react with the 15 mmoles of the acid
This will increase the concentration of salt by 15 mmoles and decrease the concentration of acid by 15 mmoles
Therefore, the resulting pH can be calculated as,
pH = pKa + log (75+15) /(100-15)
pH = 3.475 + log(90/85) = 3.49
pH = 3.49








